Objective: To investigate the safety and efficacy of a modified busulfan (Bu)/cyclophosphamide (Cy) conditioning regimen incorporating Selinexor for allogeneic hematopoietic stem cell transplantation in relapsed/refractory hematologic malignancies.
Methods: We retrospectively evaluated the clinical outcomes of patients with hematologic malignancies who had received allo-HSCT with conditioning incorporating Selinexor at the Institute of Hematology and Blood Disease Hospital.
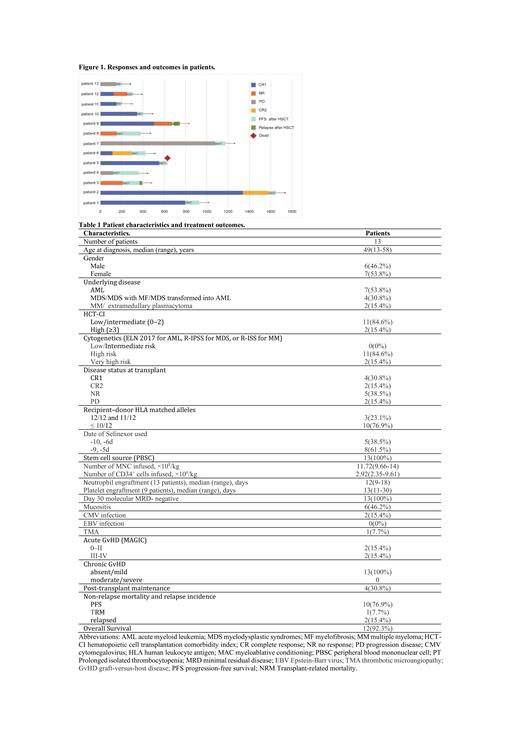
Results: Between September 2022 and June 2023, 13 adult patients with high-risk hematologic malignancies were enrolled including 6 males and 7 females with a median age of 49 (13-58) years. There were 7 patients with acute myeloid leukemia (AML), 4 with myelodysplastic syndromes (MDS)/AML, 1 with multiple myeloma (MM) and 1 with extramedullary plasmacytoma.
Six patients were in complete remission (CR), four in CR1, two in ≥CR2, five in non-remission (NR), and two patients were in a progressive disease status (PD) prior to conditioning. Three patients received matched-sibling donors transplant, while ten received related haploid hematopoietic stem cell transplantation.
All patients had received myeloablative conditioning regimen incorporating Selinexor. Eleven patients received the conditioning: Bu (3.2mg/kg/d*2d); Melphalan (Mel, 60mg/m 2/d*2d), Fludarabine(Flu 30mg/m 2/d*3d) or CLA (5mg/m 2/d*3d), Cy (40mg/kg/d*2d), Selinexor 60mg/d*2d. The other 2 patients received the conditioning: Decitabine (DAC, 20mg/m 2/d*5d), Bu (3.2mg/kg/d*3d), Cy (40mg/kg/d *2d), Flu 30mg/m2/d*3d, Selinexor 60mg/d*2d.
The median amount of infused mononuclear cell and CD34 + stem cell were 11.72×10 8/kg(range, 9.66 to 14) and 2.92×10 6/kg(range, 2.35 to 9.61), respectively. All the 13 patients achieved neutrophil engraftment with a median time of 12 (range, 9 to 18) days and 9 patients achieved platelet engraftment with a median time of 13 (range, 11 to 30) days. Four patients were still experiencing prolonged isolated thrombocytopenia.
All patients achieved complete donor chimerism and minimal residual disease (MRD) negative at 30 days post-transplantation.The cumulative incidence of cytomegalovirus (CMV) reactivation was 15.4%, while Epstein-Barr virus (EBV) reactivation was not observed. Six patients (46.2%) experienced mucosal barrier injury, comprising three cases of oral mucosal ulcer, one case of digestive tract hemorrhage, and two cases of hemorrhagic cystitis. The cumulative incidence of II-IV and III-IV acute GVHD was 23.1% and 15.4%.
The median follow-up for all the participants was 93 (40-316) days after transplantation. Only one patient was dead due to Thrombotic Microangiopathy (TMA) 78 days after transplant. One patient experienced molecular relapse 156 days after transplant, and another patient experienced hematology relapse 20 days after transplant. The two relapsed patients had received donor lymphocyte infusions after chemotherapy. The other 10 patients were still in CR with complete donor chimerism and MRD negative. The cumulative transplant-related mortality (TRM) and relapse rates are 7.7% (1 case) and 15.4% (2 cases), respectively.
Conclusions: Incorporating Selinexor into the modified BU/CY conditioning regimen for allogeneic HSCT in patients with relapsed/refractory hematologic malignancies demonstrated a favorable safety profile. Ongoing prospective trials will provide valuable insights into the potential benefits of Selinexor in patients undergoing HSCT.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal